Chemistry, 26.07.2019 15:00 victorialeona81
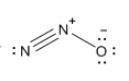
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance form is likely to contribute most to the correct structure of ? structure for nno showing three lone-pairs of electrons on the terminal nitrogen atom, a single bond between the two nitrogen atoms, a triple bond between nitrogen and oxygen, and one lone-pair of electrons on the terminal oxygen atom. structure for nno showing two lone-pairs of electrons on the terminal nitrogen atom, a double bond between the two nitrogen atoms, a double bond between nitrogen and oxygen, and two lone-pairs of electrons on the terminal oxygen atom. structure for nno showing one lone-pair of electrons on the terminal nitrogen atom, a triple bond between the two nitrogen atoms, a single bond between nitrogen and oxygen, and three lone-pairs of electrons on the terminal oxygen atom?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3

Chemistry, 23.06.2019 06:00
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 2

You know the right answer?
Which resonance form is likely to contribute most to the correct structure of n2o? which resonance...
Questions



English, 02.09.2020 18:01





Physics, 02.09.2020 18:01





English, 02.09.2020 18:01

Social Studies, 02.09.2020 18:01






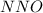 is:
is: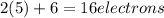
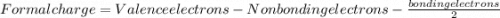
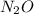 is:
is: