Chemistry, 19.09.2019 07:50 lacourboud20005
When 8.00 × 1022 molecules of ammonia react with 7.00 × 1022 molecules of oxygen according to the chemical equation shown below, how many grams of nitrogen gas are produced?
4 nh3(g) + 3 o2(g) → 2 n2(g) + 6 h2o(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 20:30
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
When 8.00 × 1022 molecules of ammonia react with 7.00 × 1022 molecules of oxygen according to the ch...
Questions










Physics, 08.08.2019 00:10



English, 08.08.2019 00:10


Mathematics, 08.08.2019 00:10





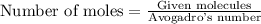 ....(1)
....(1)
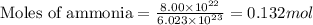
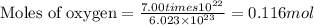
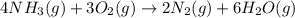
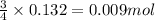 of oxygen
of oxygen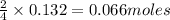 of nitrogen
of nitrogen