
Chemistry, 26.07.2019 13:30 jailenevazquez755
The solubility of agcl(s) in water at 25°c is 1.33 x 10-5 mol/l and its δh° of solution is 65.7 kj/mol. what is its solubility at 95°c?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 09:00
Look at the spectrums of a star moving towards earth and a motionless star. which of these is a correct inference that can be draw from the observation of the two spectrums? (2 points) the spectrum of a motionless star is difficult to be viewed separately using oridinary telescopes. the spectrum of a motionless star is identical to the spectrum of a star which moves towards earth. the spectrum of a star shifts towards the red region when the star moves towards earth. the spectrum of a star shifts towards the blue region when the star moves towards earth.
Answers: 2

Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
The solubility of agcl(s) in water at 25°c is 1.33 x 10-5 mol/l and its δh° of solution is 65.7 kj/m...
Questions

Mathematics, 15.04.2020 00:20

English, 15.04.2020 00:20




Biology, 15.04.2020 00:21

Health, 15.04.2020 00:21


Biology, 15.04.2020 00:21





Mathematics, 15.04.2020 00:21

Mathematics, 15.04.2020 00:21

Mathematics, 15.04.2020 00:21

Biology, 15.04.2020 00:21

Mathematics, 15.04.2020 00:21


Computers and Technology, 15.04.2020 00:21

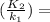 - ΔH∘ / R * ( 1 / T₂ - 1 / T₁ ) --- ( 1 )
- ΔH∘ / R * ( 1 / T₂ - 1 / T₁ ) --- ( 1 )

