

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:50
What is the composition, in atom percent, of an alloy that consists of 4.5 wt% pb and 95.5 wt% sn? the atomic weights for pb and sn are 207.19 g/mol and 118.71 g/mol, respectively.(a) 2.6 at% pb and 97.4 at% sn(b) 7.6 at% pb and 92.4 at% sn(c)97.4 at% pb and 2.6 at% sn(d) 92.4 at% pb and 7.6 at% sn
Answers: 2


Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3
You know the right answer?
If you were to assume a 95% yield for the formation of the phosphonium ion, how many milligrams of b...
Questions


Mathematics, 07.11.2019 03:31



Business, 07.11.2019 03:31


Spanish, 07.11.2019 03:31








Mathematics, 07.11.2019 03:31

English, 07.11.2019 03:31




English, 07.11.2019 03:31

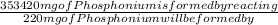 =
= 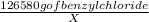
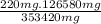
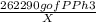
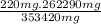
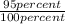 =
= 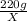
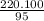 = 231.57 mg
= 231.57 mg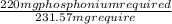 =
= 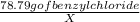
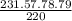 = 82.93 mg of Benzyl chloride
= 82.93 mg of Benzyl chloride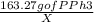
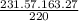 = 171.85 mg of PPh3
= 171.85 mg of PPh3

