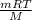Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallons that contains o2 gas at a pressure of 16,500 kpa at 23 °c. (a) what mass of o2 does the tank contain? (b) what volume would the gas occupy at stp? (c) at what temperature would the pressure in the tank equal 150.0 atm? (d) what would be the pressure of the gas, in kpa, if it were transferred to a container at 24 °c whose volume is 55.0 l?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 23:30
The sum of the oxidation numbers in a neutral compound is always
Answers: 2

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
You know the right answer?
Many gases are shipped in high-pressure containers. consider a steel tank whose volume is 55.0 gallo...
Questions

Mathematics, 08.01.2021 18:30


Social Studies, 08.01.2021 18:30


Mathematics, 08.01.2021 18:30

Mathematics, 08.01.2021 18:30


Mathematics, 08.01.2021 18:30

History, 08.01.2021 18:30

Mathematics, 08.01.2021 18:30




Business, 08.01.2021 18:30