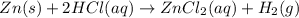Chemistry, 26.07.2019 07:30 NidaFurqan
In this reaction, what is the substance oxidized? zn(s) + 2 hcl(aq) → zncl2(aq) + h2(g) zinc chloride chlorine hydrogen zn oxygen

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of iron(fe) is 55.8 g/mol. what is the mass in grams of 2.25 moles of iron?
Answers: 1

Chemistry, 23.06.2019 11:30
How do you calculate the mass of a product when the amounts of more than one reactant are given?
Answers: 3
You know the right answer?
In this reaction, what is the substance oxidized? zn(s) + 2 hcl(aq) → zncl2(aq) + h2(g) zinc chlori...
Questions

Social Studies, 02.03.2021 02:20


Mathematics, 02.03.2021 02:20




Mathematics, 02.03.2021 02:20


Mathematics, 02.03.2021 02:20





Mathematics, 02.03.2021 02:20

Mathematics, 02.03.2021 02:20


Chemistry, 02.03.2021 02:20



Mathematics, 02.03.2021 02:20