
Chemistry, 26.09.2019 01:00 fickllyd000
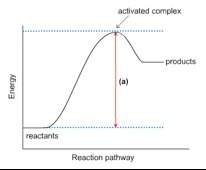
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as mercuric oxide) into liquid mercury and oxygen gas.
• write a balanced equation for the reaction.
• explain what feature is shown by the arrow labeled (a).
• using chemical symbols and dashed lines (this can be done with type), draw what the activated complex or transition state might look like.
• is this reaction exothermic or endothermic? explain.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


You know the right answer?
1. this energy diagram is for the thermal decomposition of solid mercury (ii) oxide (also known as m...
Questions

English, 01.03.2021 03:30



Social Studies, 01.03.2021 03:30

Mathematics, 01.03.2021 03:30



Arts, 01.03.2021 03:30



English, 01.03.2021 03:30


Mathematics, 01.03.2021 03:30

Mathematics, 01.03.2021 03:30

History, 01.03.2021 03:30


Mathematics, 01.03.2021 03:30


Mathematics, 01.03.2021 03:30



