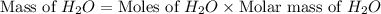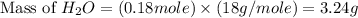Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gaseous water h2o . suppose 1.44 g of methane is mixed with 9.5 g of oxygen. calculate the maximum mass of water that could be produced by the chemical reaction. round your answer to 3 significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1
You know the right answer?
Gaseous methane ch4 will react with gaseous oxygen o2 to produce gaseous carbon dioxide co2 and gase...
Questions

Social Studies, 01.09.2019 04:00



Mathematics, 01.09.2019 04:00

Biology, 01.09.2019 04:00

Mathematics, 01.09.2019 04:00

Mathematics, 01.09.2019 04:00

English, 01.09.2019 04:00



Computers and Technology, 01.09.2019 04:00



Mathematics, 01.09.2019 04:00


Chemistry, 01.09.2019 04:00

History, 01.09.2019 04:00

Geography, 01.09.2019 04:00


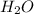 produced will be, 3.24 grams
produced will be, 3.24 grams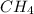 = 1.44 g
= 1.44 g
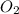 = 9.5 g
= 9.5 g
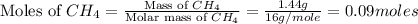
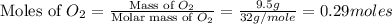
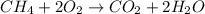
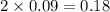 moles of
moles of