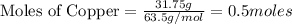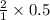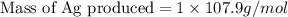Chemistry, 25.07.2019 17:30 michaellagann2020
Copper metal (cu) reacts with silver nitrate (agno3) in aqueous solution to form ag and cu(no3)2. an excess of agno3 is present. the balanced chemical equation is shown below. cu + 2agno3 mc021-1.jpg cu(no3)2 + 2ag the molar mass of cu is 63.5 g/mol. the molar mass of ag is 107.9 g/mol. what mass, in grams, of ag is produced from reaction of 31.75 g of cu? 26.95 107.9 215.91 431.82

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 17:50
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
Copper metal (cu) reacts with silver nitrate (agno3) in aqueous solution to form ag and cu(no3)2. an...
Questions

Mathematics, 09.11.2020 23:10


English, 09.11.2020 23:10


Business, 09.11.2020 23:10


Mathematics, 09.11.2020 23:10



Mathematics, 09.11.2020 23:10

Mathematics, 09.11.2020 23:10

Biology, 09.11.2020 23:10

Mathematics, 09.11.2020 23:10

Arts, 09.11.2020 23:10




History, 09.11.2020 23:10


Mathematics, 09.11.2020 23:10

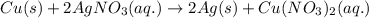
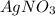 is present in excess, therefore Copper metal is considered as the limiting reagent because it limits the formation of product.
is present in excess, therefore Copper metal is considered as the limiting reagent because it limits the formation of product.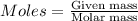 ....(1)
....(1)