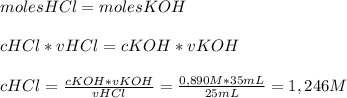Chemistry, 25.07.2019 16:00 nayelimoormann
Astudent titrates a 25 ml of an unknown concentration of hcl with 35 ml of a 0.890 m solution of koh to reach the equivalence point. what is the ph of the hcl solution? 1.2 -0.10

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 14:00
Calculate the frequency of a wave in a spring toy. the wave has a speed of 1.1 meters per second and a wavelength of 0.1 meters. *
Answers: 2

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
Astudent titrates a 25 ml of an unknown concentration of hcl with 35 ml of a 0.890 m solution of koh...
Questions

Mathematics, 17.09.2019 03:00


History, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00

Biology, 17.09.2019 03:00



Mathematics, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00


History, 17.09.2019 03:00

Mathematics, 17.09.2019 03:00


Physics, 17.09.2019 03:00

Biology, 17.09.2019 03:00