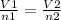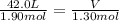Chemistry, 25.07.2019 14:00 brittany7436
Acylinder, with a piston pressing down with a constant pressure, is filled with 1.90 moles of a gas (n1), and its volume is 42.0 l (v1). if 0.600 mole of gas leak out, and the pressure and temperature remain the same, what is the final volume of the gas inside the cylinder?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
Acylinder, with a piston pressing down with a constant pressure, is filled with 1.90 moles of a gas...
Questions


Chemistry, 02.04.2021 01:00



Mathematics, 02.04.2021 01:00





Mathematics, 02.04.2021 01:00

Mathematics, 02.04.2021 01:00



Mathematics, 02.04.2021 01:00



Arts, 02.04.2021 01:00

Mathematics, 02.04.2021 01:00