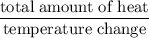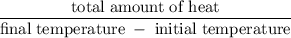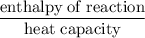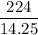Chemistry, 25.07.2019 14:00 wyattjefferds05
A6.55 g sample of aniline (c6h5nh2, molar mass = 93.13 g/mol) was combusted in a bomb calorimeter with a heat capacity of 14.25 kj/°c. if the initial temperature was 32.9°c, use the information below to determine the value of the final temperature of the calorimeter. 4 c6h5nh2(l) + 35 o2(g) → 24 co2(g) + 14 h2o(g) + 4 no2(g)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 21:00
As we move from left to right across the periodic table, what is the general trend? a) atomic radii increase. b) electronegavitiy decreases. c) nuclear shielding increases. d) metallic character decreases.
Answers: 1
You know the right answer?
A6.55 g sample of aniline (c6h5nh2, molar mass = 93.13 g/mol) was combusted in a bomb calorimeter wi...
Questions

English, 25.02.2021 05:30

French, 25.02.2021 05:30

Physics, 25.02.2021 05:30

English, 25.02.2021 05:30


Mathematics, 25.02.2021 05:30





Chemistry, 25.02.2021 05:30


Health, 25.02.2021 05:30


Chemistry, 25.02.2021 05:30

Mathematics, 25.02.2021 05:30

History, 25.02.2021 05:30

Advanced Placement (AP), 25.02.2021 05:30

Mathematics, 25.02.2021 05:30

Social Studies, 25.02.2021 05:30

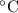 .
.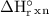 = 1.28
= 1.28 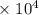 kJ.
kJ.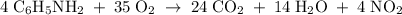
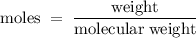
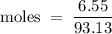
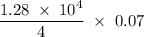
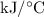 .
.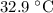 .
.