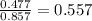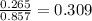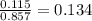Chemistry, 25.07.2019 14:00 bg988763p7cl2d
Amixture containing 0.477 mol he(g), 0.265 mol ne(g), and 0.115 mol ar(g) is confined in a 7.00-l vessel at 25 ∘c. part a calculate the partial pressure of he in the mixture.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Plz me get these answer dubble cheak ur answer plz ppl i need it right
Answers: 2

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Amixture containing 0.477 mol he(g), 0.265 mol ne(g), and 0.115 mol ar(g) is confined in a 7.00-l ve...
Questions


History, 15.04.2021 01:00

Social Studies, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00







Mathematics, 15.04.2021 01:00



Mathematics, 15.04.2021 01:00

Biology, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00


Computers and Technology, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00