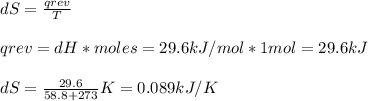Chemistry, 25.07.2019 11:00 kornut7316
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δhvap = 29.6 kj/mol. (a) when br2(l) boils at its normal boiling point, does its entropy increase or decrease? decrease (δs is negative) increase (δs is positive) (b) calculate the value of δs when 1.00 mol of br2(l) is vaporized at 58.8°c.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
You know the right answer?
The normal boiling point of bromine, br2(l), is 58.8°c, and its molar enthalpy of vaporization is δh...
Questions



History, 23.07.2019 17:00

Chemistry, 23.07.2019 17:00


Biology, 23.07.2019 17:00

Biology, 23.07.2019 17:00




Biology, 23.07.2019 17:00



Mathematics, 23.07.2019 17:00

Mathematics, 23.07.2019 17:00

History, 23.07.2019 17:00


Mathematics, 23.07.2019 17:00