
Chemistry, 25.07.2019 07:30 ramentome7542
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of mgo is formed. what is the percent yield of this reaction? mg3n2 + 3 h2o --> 2 nh3 + 3 mgo

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement is true regarding the diagram of circle p? the sum of y and z must be 2x. the sum of y and z must be x. the difference of z and y must be 2x. the difference of z and y must be x
Answers: 1
You know the right answer?
When a 3.80 g sample of magnesium nitride (mw 101g/mol) is reacted with 3.30 g of water, 3.60 g of m...
Questions


Mathematics, 18.03.2021 23:50







Mathematics, 18.03.2021 23:50



Mathematics, 18.03.2021 23:50


Mathematics, 18.03.2021 23:50

History, 18.03.2021 23:50




Mathematics, 18.03.2021 23:50

Mathematics, 18.03.2021 23:50

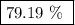 .
.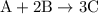
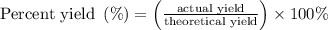 ......(1)
......(1) and is as follows:
and is as follows:
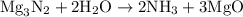 ……. (2)
……. (2)
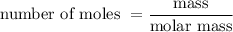
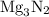 is
is 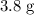 .
.
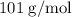 .
.
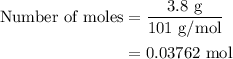
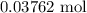 .
.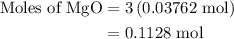
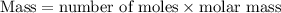
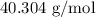 .
.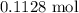 .
.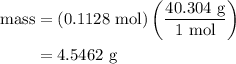
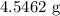 .
.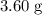 .
.
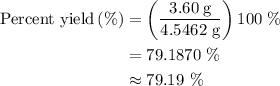
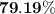 .
.


