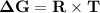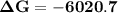Chemistry, 25.07.2019 07:00 zetrenne73
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol. calculate δg at 298 k if the partial pressures of no2 and n2o4 are 0.38atm and 1.64atm , respectively.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
Consider the reaction 2no2(g) → n2o4(g) and δg∘(no2(g)) = 51.84 kj/mol, δg∘(n2o4(g)) = 98.28 kj/mol....
Questions


Biology, 02.10.2020 14:01

World Languages, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01


Chemistry, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01

English, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Chemistry, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

English, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01