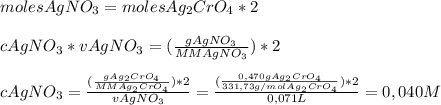We add excess na2cro4 solution to 71.0 ml of a solution of silver nitrate (agno3) to form insoluble solid ag2cro4. when it has been dried and weighed, the mass of ag2cro4 is found to be 0.470 grams. what is the molarity of the agno3 solution? answer in units of m.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1


Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3
You know the right answer?
We add excess na2cro4 solution to 71.0 ml of a solution of silver nitrate (agno3) to form insoluble...
Questions

Mathematics, 11.07.2019 18:50



Spanish, 11.07.2019 18:50



History, 11.07.2019 18:50


Physics, 11.07.2019 18:50






Arts, 11.07.2019 18:50

History, 11.07.2019 18:50



History, 11.07.2019 18:50

Chemistry, 11.07.2019 18:50