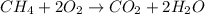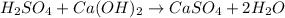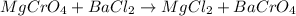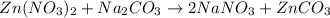Chemistry, 25.07.2019 06:30 Brittany0512
Which equation represents an oxidation-reduction reaction? 1.  ch4 + 2o2 → co2 + 2h2o 2.  h2so4 + ca(oh)2 → caso4 + 2h2o 3.  mgcro4 + bacl2 → mgcl2 + bacro4 4.  zn(no3)2 + na2co3 → 2nano3 + znco3?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Which equation represents an oxidation-reduction reaction? 1.  ch4 + 2o2 → co2 + 2h2o 2.  h2so4 +...
Questions


Social Studies, 15.12.2021 08:30







Mathematics, 15.12.2021 08:30

English, 15.12.2021 08:30



Geography, 15.12.2021 08:30


Mathematics, 15.12.2021 08:30



English, 15.12.2021 08:30