
Chemistry, 25.07.2019 03:00 ewymer3901
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid according to the equation: ai(oh)3 +3hciaici, +3h2o. determine the moles of acid neutralized if a tablet contains 0.200 mol of al(oh)3

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1


Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
Some antacid tablets contain aluminum hydroxide. the aluminum hydroxide reacts with stomach acid acc...
Questions


Chemistry, 02.10.2020 14:01



Chemistry, 02.10.2020 14:01

English, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Biology, 02.10.2020 14:01


Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01



Mathematics, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Social Studies, 02.10.2020 14:01

Chemistry, 02.10.2020 14:01

Mathematics, 02.10.2020 14:01

Computers and Technology, 02.10.2020 14:01

Biology, 02.10.2020 14:01

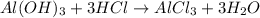
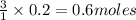 of hydrochloric acid.
of hydrochloric acid.

