
Chemistry, 24.07.2019 17:00 keilahkimbrough8
A25.0 ml sample of a 0.1700 m solution of aqueous trimethylamine is titrated with a 0.2125 m solution of hcl. calculate the ph of the solution after 10.0, 20.0, and 30.0 ml of acid have been added; pkb of (ch3)3n = 4.19 at 25°c.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 23.06.2019 13:20
In the haber reaction, patented by german chemist fritz haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. this reaction is now the first step taken to make most of the world's fertilizer. suppose a chemical engineer studying a new catalyst for the haber reaction finds that 671 liters per second of dinitrogen are consumed when the reaction is run at 271c and 0.99atm. calculate the rate at which ammonia is being produced. give your answer in kilograms per second. round your answer to significant digits.
Answers: 3
You know the right answer?
A25.0 ml sample of a 0.1700 m solution of aqueous trimethylamine is titrated with a 0.2125 m solutio...
Questions



Mathematics, 08.01.2021 04:50

Arts, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50

Biology, 08.01.2021 04:50


Mathematics, 08.01.2021 04:50




Mathematics, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50



Spanish, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50

Health, 08.01.2021 04:50

Mathematics, 08.01.2021 04:50

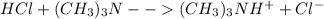
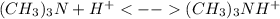
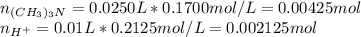

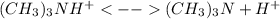 ) defines the pH via the equilibrium law of mass action calculation:
) defines the pH via the equilibrium law of mass action calculation:![Ka=\frac{[H^+]_{eq}[(CH_3)_3N]_{eq}}{[(CH_3)_3NH^+]_{eq}}](/tpl/images/0128/0009/05681.png)
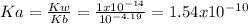
 as the concentration change during the chemical reaction, its value will account for the concentration of hydrogen which subsequently allows us to compute the pH considering that the initial concentrations match with the concentrations after the addition of the 10mL of HCl, thus:
as the concentration change during the chemical reaction, its value will account for the concentration of hydrogen which subsequently allows us to compute the pH considering that the initial concentrations match with the concentrations after the addition of the 10mL of HCl, thus: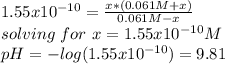
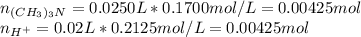

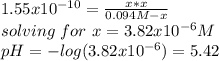

![M_{(CH_3)_3N}=\frac{0.00425mol-0.00425mol}{0.025L+0.03L} =0M\\M_{H^+}=\frac{0.006375mol-0.00425mol}{0.025L+0.03L} =0.0386MIn this case, the concentration of hydrogen in excess, 0.0386M is just enough to compute the pH since all of the base is consumed, thus:[tex]pH=-log(0.0386)=1.41](/tpl/images/0128/0009/3baeb.png)


