
Chemistry, 24.07.2019 12:30 jayowens20
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the reaction rate, tripling the concentration of y triples the rate, and doubling the concentration of z has no effect. what is the rate law for this reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1


Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1
You know the right answer?
For the reaction x2 + y + z → xy + xz, it is found that doubling the concentration of x2 doubles the...
Questions


Mathematics, 25.11.2021 16:10


Social Studies, 25.11.2021 16:10




SAT, 25.11.2021 16:10

Mathematics, 25.11.2021 16:10

Engineering, 25.11.2021 16:10


Health, 25.11.2021 16:10

Business, 25.11.2021 16:10


English, 25.11.2021 16:10


Chemistry, 25.11.2021 16:10

English, 25.11.2021 16:10


Mathematics, 25.11.2021 16:20

![\boxed{rate=k\left[ {{{\text{X}}_2}}\right]\left[ {\text{Y}} \right]}](/tpl/images/0127/2448/0a486.png) .
.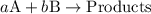
![{\text{rate}}=k{\left[{\text{A}}\right]^a}{\left[{\text{B}}\right]^b}](/tpl/images/0127/2448/dedd1.png) ...... (1)
...... (1)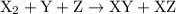
![{\text{rate}}=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}} \right]^c}](/tpl/images/0127/2448/0b279.png) …… (2)
…… (2)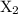 , Y, and Z respectively.
, Y, and Z respectively.![\begin{aligned}{\text{rate}}&=k{\left[{{{\text{X}}_2}}\right]^a}{\left[{\text{Y}}\right]^b}{\left[ {\text{Z}}\right]^c}\\&=k{\left[{{{\text{X}}_2}}\right]^1}{\left[ {\text{Y}}\right]^1}{\left[ {\text{Z}}\right]^0}\\&=k\left[{{{\text{X}}_2}}\right]\left[ {\text{Y}}\right]\\\end{aligned}](/tpl/images/0127/2448/fee78.png)


