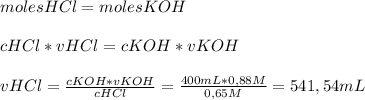Chemistry, 24.07.2019 08:00 James300102
Write the balanced equation. then calculate the volume of 0.65 m hcl required to completely neutralize 400.0 ml of 0.88 m koh.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which features are shown in the image? check all that apply. folds o anticlines o synclines o normal faults ostrike-slip faults
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 23.06.2019 01:30
The solubility of barium nitrate is 9.02 g/100 g h2o at 20°c. a 15.2 g sample of barium nitrate is added to 200.0 g of water at 20°c. is the solution saturated, unsaturated, or supersaturated? a. unsaturated b. saturated c. supersaturated
Answers: 1
You know the right answer?
Write the balanced equation. then calculate the volume of 0.65 m hcl required to completely neutrali...
Questions

Mathematics, 11.02.2021 17:10


Mathematics, 11.02.2021 17:10


Mathematics, 11.02.2021 17:10


Mathematics, 11.02.2021 17:20

History, 11.02.2021 17:20







Mathematics, 11.02.2021 17:20

Mathematics, 11.02.2021 17:20

Mathematics, 11.02.2021 17:20

Biology, 11.02.2021 17:20


Mathematics, 11.02.2021 17:20