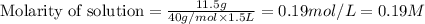Chemistry, 24.07.2019 06:00 alonzomacias1989
Calculate the molarity of a solution prepared by dissolving 11.5 g naoh in enough water to make 1.5 l of solution. 1. 7.6 m 2. 2.3 m 3. 0.19 m 4. 0.43 m

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Calculate the molarity of a solution prepared by dissolving 11.5 g naoh in enough water to make 1.5...
Questions


Computers and Technology, 27.02.2020 00:14



Social Studies, 27.02.2020 00:14


English, 27.02.2020 00:14


Mathematics, 27.02.2020 00:14






Computers and Technology, 27.02.2020 00:15





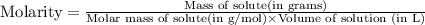
![[23+16+1]g/mol=40g/mol](/tpl/images/0126/2376/32450.png)