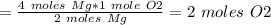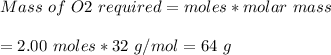Chemistry, 24.07.2019 03:30 carlinryan
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium oxide (mgo). 2mg + o2 2mgo the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 is required to react completely with 4.00 mol of mg? a)2.00 b)64.0 c)128 d)256

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium o...
Questions


Mathematics, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30


Mathematics, 07.04.2021 21:30

English, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30

English, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30


Mathematics, 07.04.2021 21:30

Chemistry, 07.04.2021 21:30


Mathematics, 07.04.2021 21:30


Mathematics, 07.04.2021 21:30

Mathematics, 07.04.2021 21:30