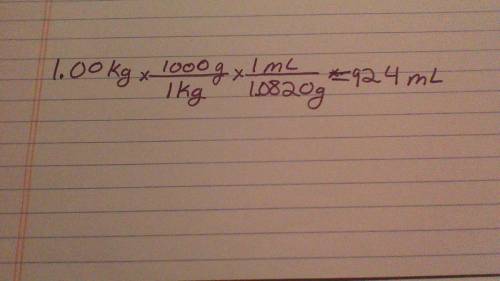Chemistry, 26.09.2019 18:00 jojojolie8570
Assuming a 100% yield what volume of acetic anhydride(density1.0820g/cm3) is required to produce 1.00kg of aspirin?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 23.06.2019 05:30
Scientist think that animals with remarkably similar embryo development probably shared a common ancestor
Answers: 1
You know the right answer?
Assuming a 100% yield what volume of acetic anhydride(density1.0820g/cm3) is required to produce 1.0...
Questions

Arts, 26.10.2020 06:00


Mathematics, 26.10.2020 06:00


Biology, 26.10.2020 06:00


Mathematics, 26.10.2020 06:00



English, 26.10.2020 06:00




Mathematics, 26.10.2020 06:00

English, 26.10.2020 06:00

History, 26.10.2020 06:00


Mathematics, 26.10.2020 06:00

Mathematics, 26.10.2020 06:00