
Chemistry, 23.07.2019 19:30 Jocelyn0925
Which of the following statements is true of combustion reactions? a. hydrogen gas (h2) must be one of the reactants b. water (h2o) is always a product c. energy is released by the reaction d. carbon monoxide (co2) is always produced

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:00
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
You know the right answer?
Which of the following statements is true of combustion reactions? a. hydrogen gas (h2) must be one...
Questions

Mathematics, 02.12.2019 04:31

History, 02.12.2019 04:31



Mathematics, 02.12.2019 04:31

English, 02.12.2019 04:31










Geography, 02.12.2019 04:31

Social Studies, 02.12.2019 04:31


Social Studies, 02.12.2019 04:31

History, 02.12.2019 04:31


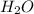 ) is always a product, is true of combustion reactions.
) is always a product, is true of combustion reactions.

