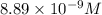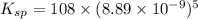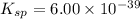Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1



Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
The molar solubility of ba3(po4)2 is 8.89 x 10-9 m in pure water. calculate the ksp for ba3(po4)2. t...
Questions




Mathematics, 09.06.2020 19:57


German, 09.06.2020 19:57


English, 09.06.2020 19:57


Biology, 09.06.2020 19:57

Mathematics, 09.06.2020 19:57

Biology, 09.06.2020 19:57

Mathematics, 09.06.2020 19:57

Chemistry, 09.06.2020 19:57




Mathematics, 09.06.2020 19:57


Chemistry, 09.06.2020 19:57

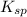 is
is 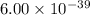
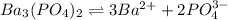
![K_{sp}=[Ba^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0124/1385/f39cc.png)
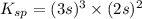
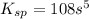
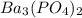 = s =
= s =