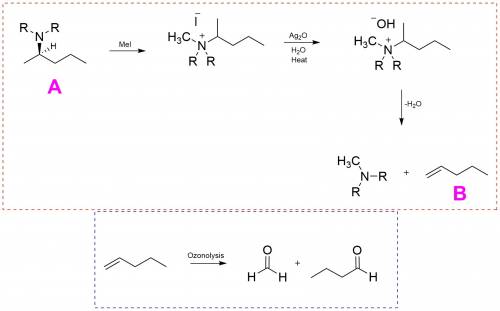
Achiral amine a having the r configuration undergoes hofmann elimination to form an alkene b as the major product. b is oxidatively cleaved with ozone, followed by ch3sch3, to form ch2═o and ch3ch2ch2cho. what are the structures of a and b? indicate stereochemistry where appropriate.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Achiral amine a having the r configuration undergoes hofmann elimination to form an alkene b as the...
Questions



Mathematics, 23.04.2021 20:30

Mathematics, 23.04.2021 20:30



Mathematics, 23.04.2021 20:30

History, 23.04.2021 20:30



Mathematics, 23.04.2021 20:30



History, 23.04.2021 20:30



Health, 23.04.2021 20:30


English, 23.04.2021 20:30