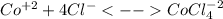In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(aq) is pink and cocl42-(aq) is blue. at low temperature the pink color predominates. at high temperature the blue color is strong. if we represent the equilibrium as: +(aq) + 4cl-(aq) cocl42-(aq) we can conclude that: 1. this reaction is: a. exothermic b. endothermic c. neutral d. more information is needed to answer this question.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
In an aqueous chloride solution cobalt(ii) exists in equilibrium with the complex ion cocl42-. co2+(...
Questions

Mathematics, 16.10.2020 15:01

History, 16.10.2020 15:01


Mathematics, 16.10.2020 15:01

Social Studies, 16.10.2020 15:01

English, 16.10.2020 15:01

English, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Health, 16.10.2020 15:01

Advanced Placement (AP), 16.10.2020 15:01

English, 16.10.2020 15:01


Social Studies, 16.10.2020 15:01



Physics, 16.10.2020 15:01


Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01