Chemistry, 23.07.2019 16:30 sandlobster3129
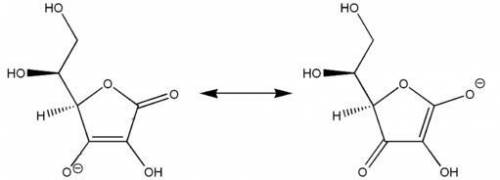
For resonance forms of a molecule or ion, a. one always corresponds to the observed structure b. all the resonance structures are seen in various proportions c. the observed structure is an average of the resonance forms d. the same atoms need not be bonded to each other e. there cannot be more than two resonance structures for a given species

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2


Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3
You know the right answer?
For resonance forms of a molecule or ion, a. one always corresponds to the observed structure b. al...
Questions

Mathematics, 24.04.2020 09:27


Mathematics, 24.04.2020 09:28

Mathematics, 24.04.2020 09:28


Mathematics, 24.04.2020 09:28

History, 24.04.2020 09:28

History, 24.04.2020 09:28


Mathematics, 24.04.2020 09:30

Mathematics, 24.04.2020 09:30

Mathematics, 24.04.2020 09:31

Mathematics, 24.04.2020 09:31

History, 24.04.2020 09:51

Geography, 24.04.2020 09:51


Business, 24.04.2020 09:51



Mathematics, 24.04.2020 09:51