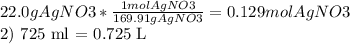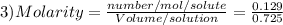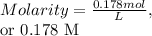Chemistry, 23.07.2019 16:00 agilitygirl1
After a chemistry student made a agno3(small 3 at bottom) solution., she wanted to determine the molar concentration of it. if 22.0g of agno3(small 3) (molar mass=169.91 g/mol) were dissolved in water to a final solution volume of 725 ml, what is the molar concentration of this solution.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 23.06.2019 02:00
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2
You know the right answer?
After a chemistry student made a agno3(small 3 at bottom) solution., she wanted to determine the mol...
Questions



Mathematics, 01.12.2021 05:50




Mathematics, 01.12.2021 05:50

Mathematics, 01.12.2021 05:50




Biology, 01.12.2021 05:50

Mathematics, 01.12.2021 05:50

Chemistry, 01.12.2021 05:50


Advanced Placement (AP), 01.12.2021 05:50

Health, 01.12.2021 05:50



Mathematics, 01.12.2021 05:50