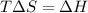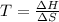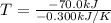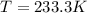Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1



Chemistry, 23.06.2019 01:30
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2
You know the right answer?
If δh = -70.0 kj and δs = -0.300 kj/k , the reaction is spontaneous below a certain temperature. cal...
Questions

Social Studies, 14.07.2019 10:10




Social Studies, 14.07.2019 10:10

Mathematics, 14.07.2019 10:10

Social Studies, 14.07.2019 10:10



Mathematics, 14.07.2019 10:10








Physics, 14.07.2019 10:10

Mathematics, 14.07.2019 10:10

Geography, 14.07.2019 10:10

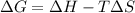
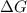 = Gibbs free energy
= Gibbs free energy 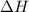 = enthalpy change = -70 kJ
= enthalpy change = -70 kJ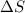 = entropy change = -0.300 kJ/K
= entropy change = -0.300 kJ/K