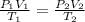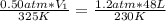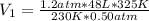Chemistry, 23.07.2019 10:30 carlydays3331
Ihave an unknown volume of gas at a pressure of 0.50 atm a temperature of 325 k. if i raise the pressure to 1.2 atm, decrease the temperature to 230 k, and measure the final volume to be 48 liters, what was the initial volume of the gas

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2


Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Ihave an unknown volume of gas at a pressure of 0.50 atm a temperature of 325 k. if i raise the pres...
Questions

History, 18.07.2019 00:20






Mathematics, 18.07.2019 00:30





Mathematics, 18.07.2019 00:30

Mathematics, 18.07.2019 00:30





Mathematics, 18.07.2019 00:30


Mathematics, 18.07.2019 00:30