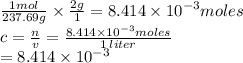Chemistry, 23.07.2019 06:00 helpmeplzandty
If you dissolve 2.00 g of nicl2*6h20 into a beaker of water and the final volume is 1.00 liters what will be the molar concentration of the solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
If you dissolve 2.00 g of nicl2*6h20 into a beaker of water and the final volume is 1.00 liters what...
Questions





Medicine, 19.06.2020 02:57

Mathematics, 19.06.2020 02:57

Mathematics, 19.06.2020 02:57

Geography, 19.06.2020 02:57

Mathematics, 19.06.2020 02:57



Mathematics, 19.06.2020 02:57


Mathematics, 19.06.2020 02:57



Geography, 19.06.2020 02:57


Biology, 19.06.2020 02:57