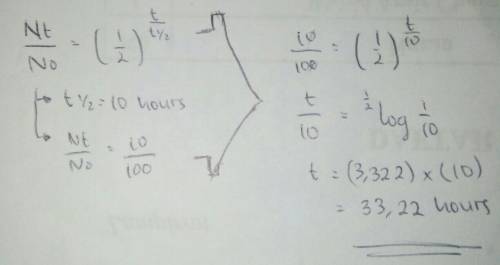Chemistry, 23.07.2019 04:00 pattydixon6
Hydrogen peroxide, which decomposes in a first order reaction, has a half-life of 10 hours in air. how long will it take for hydrogen peroxide to decompose to 10% of its original concentration?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
Hydrogen peroxide, which decomposes in a first order reaction, has a half-life of 10 hours in air. h...
Questions



History, 05.05.2020 19:34

Mathematics, 05.05.2020 19:34

Mathematics, 05.05.2020 19:34


English, 05.05.2020 19:34


Chemistry, 05.05.2020 19:34

Mathematics, 05.05.2020 19:34





English, 05.05.2020 19:34

History, 05.05.2020 19:34


History, 05.05.2020 19:35

Mathematics, 05.05.2020 19:35

Mathematics, 05.05.2020 19:35