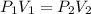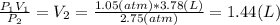Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 05:30
Calculate the temperature rise when 0.2g of propane is used to heat 400cm cubed of water.
Answers: 3

Chemistry, 23.06.2019 10:30
Can anyone explain 1. review your spectrometry data and use the known elements to identify the star's composition. which unknown elements make up this star? justify your element selections. 2. in parts i and ii of the lab, what happened to the electrons of each element to produce the different colors of light? explain your answers using important terms from the lesson and information provided in the laboratory. 3. stars composed of heavier (more massive) elements are often slightly older than stars made predominantly from hydrogen and helium. based on your data, is the newly discovered star a younger star? explain your answer.
Answers: 2
You know the right answer?
The volume of a balloon containing an ideal gas is 3.78 l at 1.05 atm pressure. what would the volum...
Questions



Mathematics, 19.07.2019 10:40





Physics, 19.07.2019 10:40






Social Studies, 19.07.2019 10:40



Social Studies, 19.07.2019 10:40

Mathematics, 19.07.2019 10:40

Physics, 19.07.2019 10:40

History, 19.07.2019 10:40

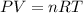

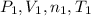 ) and changes to a second state with a final pressure, volume, molar amount and temperature (
) and changes to a second state with a final pressure, volume, molar amount and temperature (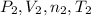 ). As we know, R is the Ideal Gas Constant and do not change with the state changes, then it is possible to obtain the equation:
). As we know, R is the Ideal Gas Constant and do not change with the state changes, then it is possible to obtain the equation: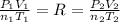
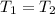 and
and 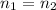 and replacing in the previews equation we obtain:
and replacing in the previews equation we obtain: