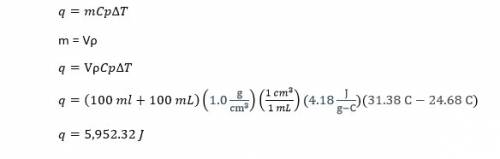In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both solutions were originally at 24.68c. after the reaction, the final temperature is 31.38c. assuming that all the solutions have a density of 1.0 g/cm3 and a specific heat capacity of 4.18 j/8c ? g, calculate the enthalpy change for the neutralization of hcl by naoh

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1


Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
In a coffee-cup calorimeter, 100.0 ml of 1.0 m naoh and 100.0 ml of 1.0 m hcl are mixed. both soluti...
Questions




Computers and Technology, 11.12.2019 04:31

Mathematics, 11.12.2019 04:31





Computers and Technology, 11.12.2019 04:31

Chemistry, 11.12.2019 04:31


Biology, 11.12.2019 04:31

English, 11.12.2019 04:31