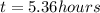How long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous cu2+ ions to produce 5.00 moles of copper metal? how long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous cu2+ ions to produce 5.00 moles of copper metal? 5.36 hours 2.68 hours 0.373 hours 0.187 hours?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 09:30
The earth's surface is (science) a: studied using seismic waves b: constantly changing over time c: only studied indirectly d: the same today as million of years
Answers: 1

Chemistry, 23.06.2019 11:30
Which of the following is a property of an acid solution? a. slippery to the touch b. ph less than 7 c. turns red litmus paper blue d. bitter taste
Answers: 1
You know the right answer?
How long must a constant current of 50.0 a be passed through an electrolytic cell containing aqueous...
Questions

Physics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Computers and Technology, 11.09.2020 22:01

Social Studies, 11.09.2020 22:01

Social Studies, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Spanish, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Physics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Biology, 11.09.2020 22:01

Mathematics, 11.09.2020 22:01

Social Studies, 11.09.2020 22:01

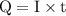
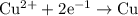
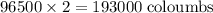
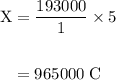
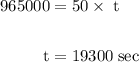
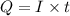
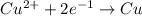
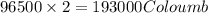 of electricity deposits 1 mole of Cu.
of electricity deposits 1 mole of Cu.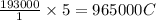
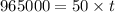
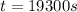 (1 hour=3600sec)
(1 hour=3600sec)