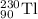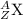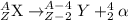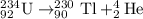Chemistry, 22.07.2019 19:30 casandraserrat375
When the nuclide uranium-234 undergoes alpha decay: the name of the product nuclide is . the symbol for the product nuclide is?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:20
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1


Chemistry, 22.06.2019 14:00
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

You know the right answer?
When the nuclide uranium-234 undergoes alpha decay: the name of the product nuclide is . the symbol...
Questions

Chemistry, 31.03.2021 05:10



History, 31.03.2021 05:10



Mathematics, 31.03.2021 05:10

Mathematics, 31.03.2021 05:10




Mathematics, 31.03.2021 05:10


Biology, 31.03.2021 05:10


Mathematics, 31.03.2021 05:10

Chemistry, 31.03.2021 05:10

Mathematics, 31.03.2021 05:10

Mathematics, 31.03.2021 05:10

Mathematics, 31.03.2021 05:10