
Chemistry, 22.07.2019 15:00 katelynnjoyce1
Hydrogen gas reacts with oxygen to form water. 2h2( g.+o2( g.→2h2o( g.δh=−483.5kj determine the minimum mass of hydrogen gas required to produce 216 kj of heat.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1
You know the right answer?
Hydrogen gas reacts with oxygen to form water. 2h2( g.+o2( g.→2h2o( g.δh=−483.5kj determine the mini...
Questions


Mathematics, 25.09.2019 03:30


Business, 25.09.2019 03:30

Geography, 25.09.2019 03:30


English, 25.09.2019 03:30


Social Studies, 25.09.2019 03:30


Mathematics, 25.09.2019 03:30

History, 25.09.2019 03:30


Health, 25.09.2019 03:30

Mathematics, 25.09.2019 03:30

Mathematics, 25.09.2019 03:30

Computers and Technology, 25.09.2019 03:30

Health, 25.09.2019 03:30


Health, 25.09.2019 03:30

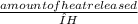 x 2 mol H₂
x 2 mol H₂

