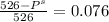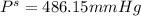Chemistry, 22.07.2019 11:30 ericasolis2614
What is the vapor pressure of a 45.0 % solution of glucose, c6h12o6, at 90.0°c, given that the vapor pressure of pure water at that temperature is 526 mm hg?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
What is the vapor pressure of a 45.0 % solution of glucose, c6h12o6, at 90.0°c, given that the vapor...
Questions




Health, 06.06.2021 09:10

World Languages, 06.06.2021 09:20

English, 06.06.2021 09:20

Biology, 06.06.2021 09:20




Mathematics, 06.06.2021 09:20

English, 06.06.2021 09:20

Computers and Technology, 06.06.2021 09:20

Mathematics, 06.06.2021 09:20

Mathematics, 06.06.2021 09:20

Mathematics, 06.06.2021 09:20

Mathematics, 06.06.2021 09:20

Mathematics, 06.06.2021 09:20


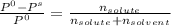 = 0.25 / (0.25 + 3.05)
= 0.25 / (0.25 + 3.05)