
Chemistry, 22.07.2019 11:30 RickandMorty420710
Consider the reaction of zn metal with hydrochloric acid: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) if 2.57 g of zn is reacted with 0.500 moles of hcl in a 3.00 l container what pressure does the generated h2 exert against the container walls at 35.8 ℃?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
This line graph compares the growth of plants that were kept in the sun for different amounts of time.a) on day 7, the plants kept in the sun for 3 hours were how tall? b) on day 7, the plants kept in the sun for 6 hours were how tall? c) on day 10, the plants kept in the sun for 9 hours were how tall? d) on day 11, the plant that was grown with 1 hour of sunlight was how tall? e) based on the graph, the plant grows best in what amount of sunlight?
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1


Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Consider the reaction of zn metal with hydrochloric acid: zn(s) + 2hcl(aq) → zncl2(aq) + h2(g) if 2...
Questions

Mathematics, 30.06.2021 23:30



Physics, 30.06.2021 23:30

Mathematics, 30.06.2021 23:30

Engineering, 30.06.2021 23:30


Arts, 30.06.2021 23:30

Mathematics, 30.06.2021 23:30

English, 30.06.2021 23:30


Biology, 30.06.2021 23:30

Arts, 30.06.2021 23:30

Mathematics, 30.06.2021 23:30

Mathematics, 30.06.2021 23:30

Mathematics, 30.06.2021 23:30

Mathematics, 30.06.2021 23:30

English, 30.06.2021 23:30



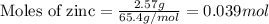
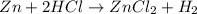
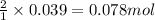 of HCl
of HCl of hydrogen gas
of hydrogen gas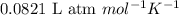
![35.8^oC=[35.8+273]K=308.8K](/tpl/images/0119/3926/fdfeb.png)



