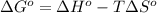Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

You know the right answer?
Acetylene, c2h2, has a standard enthalpy of formation, δh° = 226.7 kj/mol, and a standard entropy ch...
Questions



Mathematics, 26.03.2020 23:42

History, 26.03.2020 23:42






Mathematics, 26.03.2020 23:42

English, 26.03.2020 23:42

Business, 26.03.2020 23:42



English, 26.03.2020 23:42

History, 26.03.2020 23:42




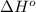 = 226.7 kJ/K mol,
= 226.7 kJ/K mol, 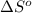 = 58.8 J/mol =
= 58.8 J/mol = 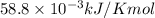
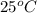 = (25 + 273) K = 298 K
= (25 + 273) K = 298 K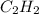 ) as follows.
) as follows.