
Chemistry, 21.07.2019 23:30 klslaughter07
Suppose a student titrates a 10.00-ml aliquot of saturated ca(oh)2 solution to the equivalence point with 16.08 ml of 0.0199 m hcl. what was the initial [oh − ]?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3
You know the right answer?
Suppose a student titrates a 10.00-ml aliquot of saturated ca(oh)2 solution to the equivalence point...
Questions


Mathematics, 11.06.2020 16:57



Mathematics, 11.06.2020 16:57

English, 11.06.2020 16:57



Physics, 11.06.2020 16:57


Chemistry, 11.06.2020 16:57

Geography, 11.06.2020 16:57


Mathematics, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57

Mathematics, 11.06.2020 16:57


![\frac{number of moles of [OH-]}{volume of solution (L)}](/tpl/images/0117/3733/f0ec8.png) =
= 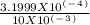 = 0.03199 M
= 0.03199 M

