
Chemistry, 21.07.2019 23:00 dajeourcrazy15
A5.018 gram sample of a certain hydrate of magnesium sulfate, mgso4•xh2o, is heated until all the water is driven off. the resulting anhydrous compound weighs 2.449 grams. what is the formula of the hydrate?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1

Chemistry, 23.06.2019 13:00
Sort these isotopes by whether they are most likely to undergo fusion or fission. hydrogen-3, uranium-233, plutonium-239, hydrogen-1, helium-3, plutonium-241
Answers: 2
You know the right answer?
A5.018 gram sample of a certain hydrate of magnesium sulfate, mgso4•xh2o, is heated until all the wa...
Questions


Mathematics, 12.02.2021 14:00


Mathematics, 12.02.2021 14:00


Chemistry, 12.02.2021 14:00





Engineering, 12.02.2021 14:00


History, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00

Mathematics, 12.02.2021 14:00


Medicine, 12.02.2021 14:00


English, 12.02.2021 14:00

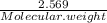
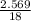
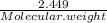

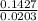 = 7.02 ~7
= 7.02 ~7

