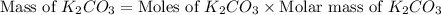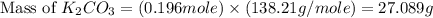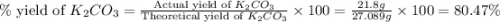Chemistry, 21.07.2019 23:00 asuhdude57
)determine the theoretical yield and the percent yield if 21.8 g of k2co3 is produced from reacting 27.9 g ko2 with 29.0 l of co2 (at stp). the molar mass of ko2 = 71.10 g/mol and k2co3 = 138.21 g/mol.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 01:30
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

You know the right answer?
)determine the theoretical yield and the percent yield if 21.8 g of k2co3 is produced from reacting...
Questions





Social Studies, 07.11.2019 00:31






English, 07.11.2019 00:31





Mathematics, 07.11.2019 00:31


Social Studies, 07.11.2019 00:31


Mathematics, 07.11.2019 00:31

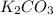 = 27.089 g
= 27.089 g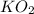 = 27.9 g
= 27.9 g
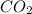 = 29.0 L (At STP)
= 29.0 L (At STP)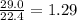 mole of
mole of 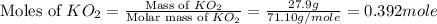
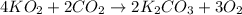
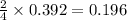 moles of
moles of