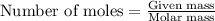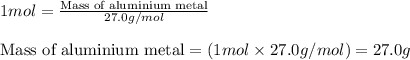Chemistry, 21.08.2019 11:30 deanlmartin
Aluminum metal reacts with hcl to produce aluminum chloride and hydrogen gas. how many grams of aluminum metal must be added to an excess of hcl to produce 33.6 l of hydrogen gas, if the gas is at stp?
(a) 18.0 g
(b) 35.0 g
(c) 27.0 g
(d) 4.50 g
(e) 9.00 g

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 16:30
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
Aluminum metal reacts with hcl to produce aluminum chloride and hydrogen gas. how many grams of alum...
Questions


Mathematics, 18.04.2021 23:00


Biology, 18.04.2021 23:00

History, 18.04.2021 23:00




English, 18.04.2021 23:00

History, 18.04.2021 23:00



Health, 18.04.2021 23:00





English, 18.04.2021 23:00

Mathematics, 18.04.2021 23:00

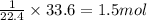 of hydrogen gas
of hydrogen gas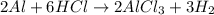
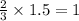 mole of aluminium metal
mole of aluminium metal