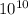Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 15:30
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2

Chemistry, 23.06.2019 18:10
A1 forms when an acid is neutralized by a base. 1. salts can be neutral, or in solutions. salts of 2. strong acid–strong base reactions produce solutions with 3. water. salts formed from the neutralization of weak acids or weak 4. bases water. they produce solutions that are acidic or . basic. for example, the ph of a solution at the equivalence point is . greater than for a acid titration. solutions that resist changes in ph are called solutions. the buffer is the amount of acid or base that can be added to a buffer without changing the ph greatly.
Answers: 1

Chemistry, 23.06.2019 19:00
1. give an example of a possible hypothesis involving food. 2. what is a simple experiment that you could conduct to test your hypothesis?
Answers: 1
You know the right answer?
Calculate the equilibrium constant for the reaction between fe2+(aq) and zn(s) under standard condit...
Questions


Mathematics, 24.08.2019 06:30



Physics, 24.08.2019 06:30

Mathematics, 24.08.2019 06:30

Mathematics, 24.08.2019 06:30



Social Studies, 24.08.2019 06:30

English, 24.08.2019 06:30

Advanced Placement (AP), 24.08.2019 06:30

Mathematics, 24.08.2019 06:30

Biology, 24.08.2019 06:30

History, 24.08.2019 06:30





Mathematics, 24.08.2019 06:30

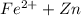 → Fe +
→ Fe + 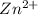
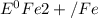 = standard reduction potential of Fe2+/Fe = -0.44 v
= standard reduction potential of Fe2+/Fe = -0.44 v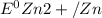 = standard reduction potential of Zn2+/Zn = -0.763 v
= standard reduction potential of Zn2+/Zn = -0.763 v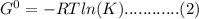
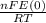 )= exp (
)= exp (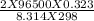 ) = 8.46 x
) = 8.46 x