
Chemistry, 21.07.2019 14:00 angel234wilcox
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction caco3(s)→cao(s)+co2(g) what is the mass of calcium carbonate needed to produce 61.0 l of carbon dioxide at stp? express your answer with the appropriate units?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2
You know the right answer?
When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reac...
Questions






Mathematics, 17.05.2021 02:50

Mathematics, 17.05.2021 02:50

Physics, 17.05.2021 02:50


Mathematics, 17.05.2021 02:50


Mathematics, 17.05.2021 02:50

Biology, 17.05.2021 02:50


Mathematics, 17.05.2021 02:50

History, 17.05.2021 02:50


Mathematics, 17.05.2021 02:50

History, 17.05.2021 02:50

Mathematics, 17.05.2021 02:50

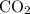 , 227.23 grams of
, 227.23 grams of 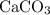 . is required.
. is required.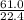
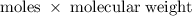
 100
100

