

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 01:40
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide as shown below. caco3(s) cao(s) + co2(g) the kp for this reaction is 1.16 at 800°c. a 5.00 l vessel containing 10.0 g of caco3(s) was evacuated to remove the air, sealed, and then heated to 800°c. ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
Answers: 1

Chemistry, 23.06.2019 02:30
Ascientist wants to know how individual lions within a pride interact with each other in their own environment. to do this, the scientist sedates and tags all of the lions within a pride. then, he places several remotely-controlled video cameras near the lions' den and performs an observational field study. he collects continuous video footage over the span of one year, analyzes the video, and then forms conclusions based on his observations.
Answers: 2

Chemistry, 23.06.2019 11:30
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
How many kj of heat are needed to completely vaporize 1.30 moles of h2o? the heat of vaporization f...
Questions


Mathematics, 29.07.2019 08:40

Physics, 29.07.2019 08:40


History, 29.07.2019 08:40


Mathematics, 29.07.2019 08:40


Mathematics, 29.07.2019 08:40


Mathematics, 29.07.2019 08:40

History, 29.07.2019 08:40

Mathematics, 29.07.2019 08:40


Health, 29.07.2019 08:40


History, 29.07.2019 08:40



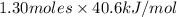
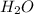 .
.

